Updated CDC Contraception Guidelines
On August 6, 2024, the CDC released the 2024 U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) and the 2024 U.S. Selected Practice Recommendations for Contraceptive Use (U.S. SPR). These documents provide current evidence-based contraception recommendations for health care providers. The goals of the U.S. MEC and the U.S. SPR are to support the provision of person-centered contraceptive counseling and services in a non-coercive manner and to remove unnecessary medical barriers to accessing and using contraception. Pharmacists should utilize these guidelines when providing birth control services.
New Guidance Includes:
- New recommendations for persons with chronic kidney disease with nephrotic syndrome, on hemodialysis, or on peritoneal dialysis.
- Updated recommendations for persons with the following personal characteristics or medical conditions: breastfeeding, postpartum, postabortion, obesity, major surgery, deep venous thrombosis or pulmonary embolism with or without anticoagulant therapy, thrombophilia, superficial venous thrombosis, valvular heart disease, peripartum cardiomyopathy, systemic lupus erythematosus, cirrhosis, liver tumor, sickle cell disease, and solid organ transplantation.
- Recommendations on additional contraceptive methods, including a vaginal pH modulator and new doses or formulations of combined oral contraceptives, contraceptive patches, vaginal rings, progestin-only pills, and levonorgestrel intrauterine devices.
New Guidance Includes:
- New recommendation for testosterone use and risk of pregnancy among transgender, gender diverse, and non-binary persons with a uterus.
- Updated recommendations for provision of medications for IUD placement and for management of bleeding irregularities during implant use.
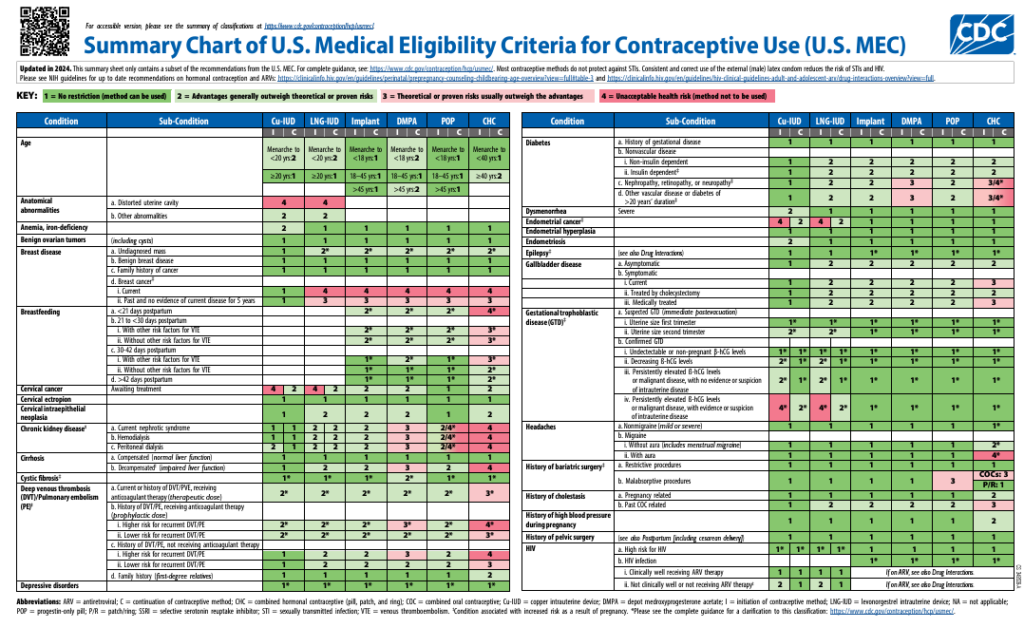
Save the updated MEC chart for a concise summary of recommendations to use during patient encounters.
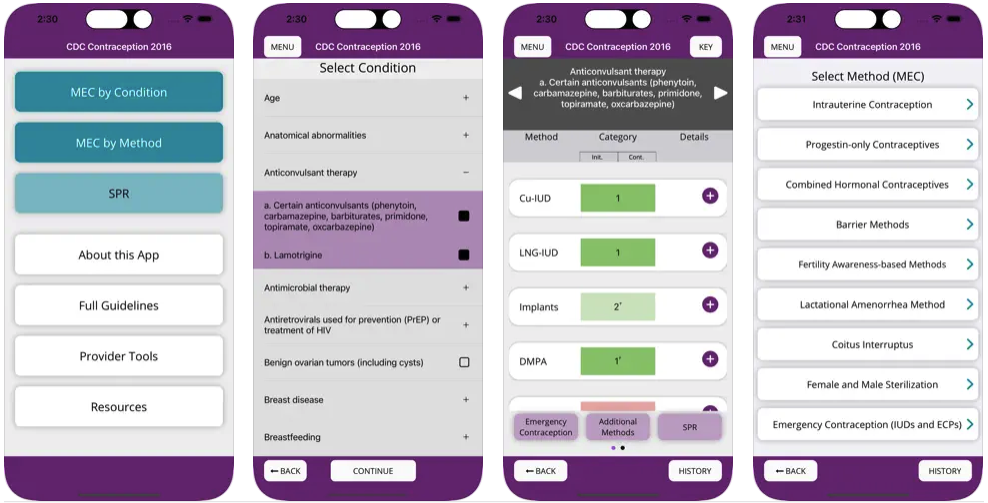
Download the updated app including updated recommendations from the 2024 U.S. MEC and U.S. SPR.
Webinar with more details coming soon!
We will be hosting a live webinar with continuing pharmacy education credit to review the updated guidelines. Look out for webinar details and registration.