Posts Tagged ‘contraception’
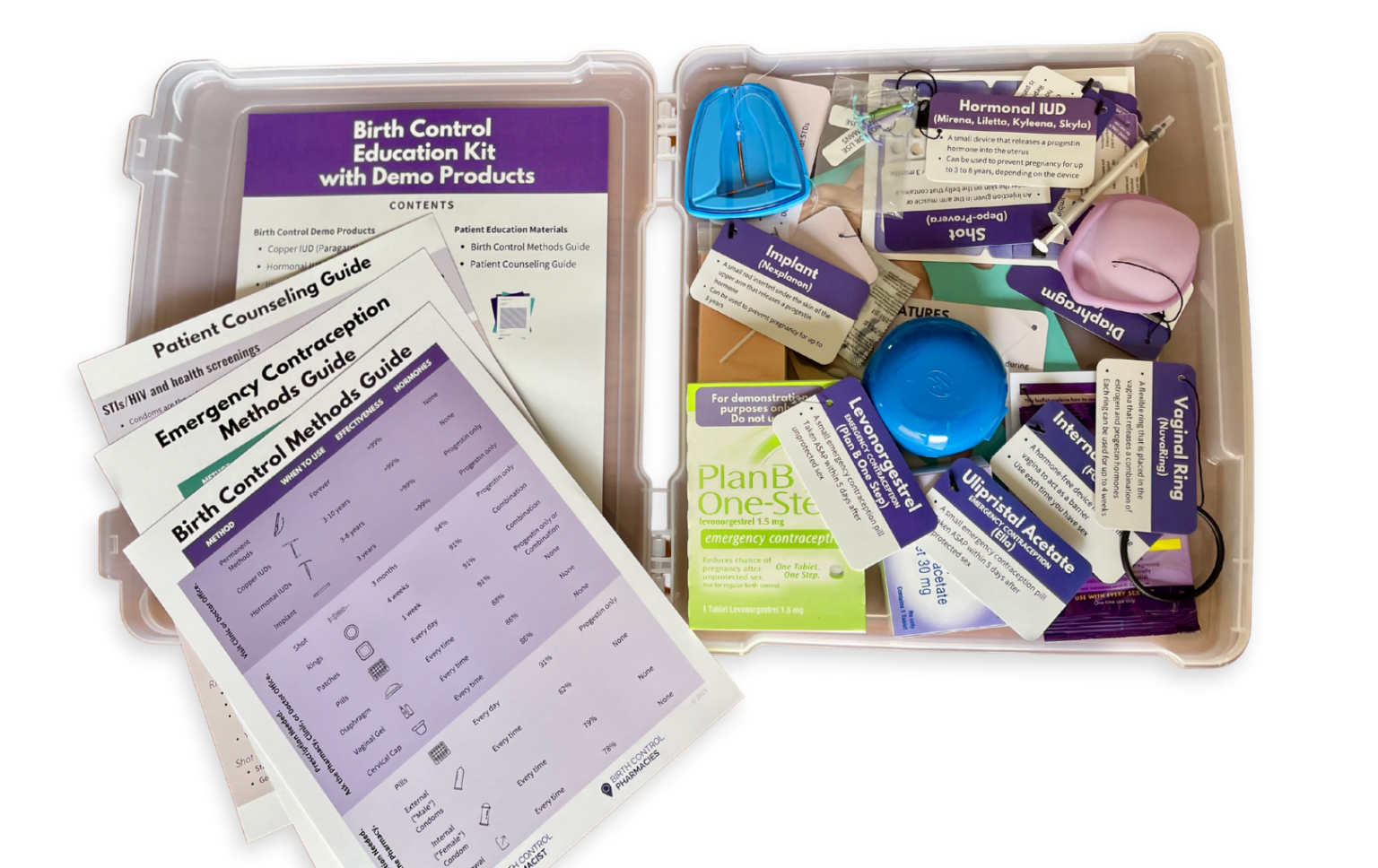
Enhancing Patient Understanding With Contraceptive Demonstration Kits
Dr. Ashley Meredith discusses how she uses the Birth Control Education Kit as a useful resource for patient counseling and engaging classroom instruction.
Read MoreContinuing Contraception Care After Trump’s Orders
In light of recent actions over the last two weeks with our new administration, I wanted to provide updates and guidance to my pharmacist peers regarding reproductive health care. On…
Read MoreFounder Reflections on 2023
As I reflect on this past year, 2023 finally brought us some wins in reproductive health. And I personally had the opportunity to contribute to landmark steps forward. 
Updated Report on State Policy Efforts to Expand Access to Contraception in Pharmacies
Download free 22-page report describing the current landscape of direct access to contraception in pharmacies, state policy approaches and experiences, as well as implementation.
Read MoreOutreach Initiative to Expand Access to Depot Medroxyprogesterone Acetate (DMPA)
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Read MoreWebinar Introduces Pharmacists to New Hormonal Contraceptives
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Read MoreWebinar Equips Pharmacists to Provide Contraception Care During COVID-19
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Read MoreNew Webinar Prepares Pharmacists to Provide Reproductive Health Services and Referrals
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Read MoreMeet Phexxi – A New Non-Hormonal Contraceptive Gel
About the Product Lactic acid, citric acid, and potassium bitartrate (Phexxi, Evofem Biosciences) is a prescription combination, non-hormonal contraceptive gel approved by the FDA in May 2020. The vaginal gel…
Read MoreMeasuring Blood Pressure: An Important Prerequisite to Prescribing Hormonal Contraception
Why is it important to measure blood pressure before prescribing hormonal contraception? Combined hormonal contraceptives (CHCs) are a relatively safe and effective method for your patients in preventing pregnancy and…
Read More