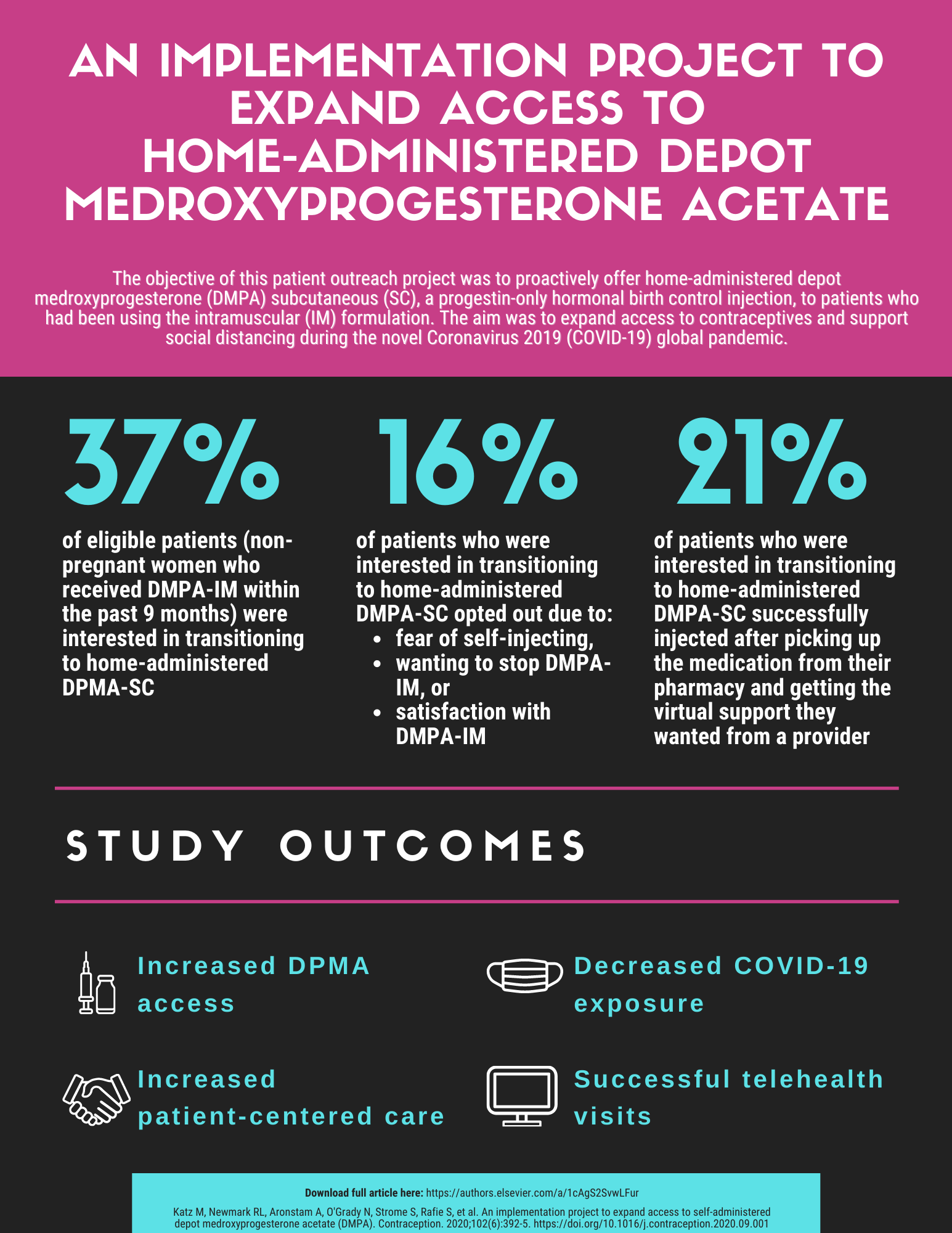
Outreach Initiative to Expand Access to Depot Medroxyprogesterone Acetate (DMPA)
Why Expand Access to DMPA
The novel coronavirus 2019 (COVID-19) presented many downstream challenges in healthcare throughout 2020. An area of particular interest was the impact COVID-19 had on access to hormonal contraception, specifically DMPA intramuscular (DMPA-IM). Prior to the pandemic, patients who used DMPA-IM would attend approximately four clinic visits per year (every 12 weeks) to obtain their injection from a medical professional.
As public health risks continued to be a primary concern and shelter-in-place orders were issued, the Centers for Medicare & Medicaid Services (CMS) issued waivers under section 1135 of the Social Security Act, which permitted state governments to adjust their public health responses to the pandemic as they deemed fit. One way Medi-Cal used this waiver was to cover self-administered DMPA subcutaneous (DMPA-SC) without a prior authorization so patients could continue using their preferred method of hormonal contraception while decreasing exposure to COVID-19 until further notice.
Our study explored interest in at-home, self-administered DMPA-SC among patients who had been on DMPA-IM at an urban, hospital-based safety-net primary care clinic in San Francisco.
Approach to Patient Outreach
Our team consisted of medical and pharmacy students, pharmacists, and medical doctors. We identified patients who had been on DMPA-IM within the last nine months (August 2019-May 2020) by searching the clinic’s electronic medical records. Through this process, we identified 90 patients and successfully reached 70 patients (78%) by telephone. Our patients were all on Medi-Cal or Family PACT. Additionally, our patient base was largely non-English speaking, so interpretative services were utilized for effective, patient-preferred communication. Once each patient’s identity was confirmed, we explained self-administered DMPA-SC and answered any questions posed by the patient. If the patient expressed interest in DMPA-SC, we ordered a prescription to their community pharmacy and offered telehealth appointments to answer any further questions, demonstrate how to self-inject, and/or observe the patient as they self-administered. Of the 70 patients reached, 26 patients (37%) were interested in learning more about DMPA-SC. By the end of our study, 15 patients (21%) successfully self-administered DMPA-SC or had a family member or friend do it for them.
Clinical Implications
Feedback received from patients previously utilizing DMPA-IM suggests at-home administration of DMPA-SC is a viable option when selecting a hormonal contraceptive. By continuing to advocate for at-home administration, the medical community can help expand access to hormonal contraception for all patients.
Check out the full study here!
References
- Depo-SubQ Provera 104 prescribing information. Pfizer, December 2020. Link. Accessed February 25, 2021.
- Katz M, Newmark RL, Aronstam A, O’Grady N, Strome S, Rafie S, et al. An implementation project to expand access to self-administered depot medroxyprogesterone acetate (DMPA). Contraception. 2020;102(6):392-5. DOI

About the Author
Sara Strome, PharmD Candidate is a pharmacy student in the Class of 2022 at the University of California San Francisco School of Pharmacy.