About Medication Abortion
About Medication Abortion
Medication abortion was approved by the FDA in 2000 and has become the most common method of abortion. On January 3, 2023, the FDA updated the mifepristone Risk Evaluation and Mitigation Strategy (REMS) allowing for certified prescribers to send prescriptions to certified pharmacies. The updated REMS will now allow brick-and-mortar, mail-order, online, local, and chain pharmacies to seek certification to stock and dispense mifepristone.
The combination of mifepristone and misoprostol for medication abortion is incredibly safe. Since 2000, more than 4.9 million patients have had a medication abortion, with less than 0.5% resulting in adverse outcomes, making it safer than several over-the-counter drugs such as ibuprofen or acetaminophen and other prescriptions like Viagra or Accutane. Today, more than 63% of all abortions in the U.S. are administered by medication.
As of 2023, medication abortions account for the majority of all US abortions
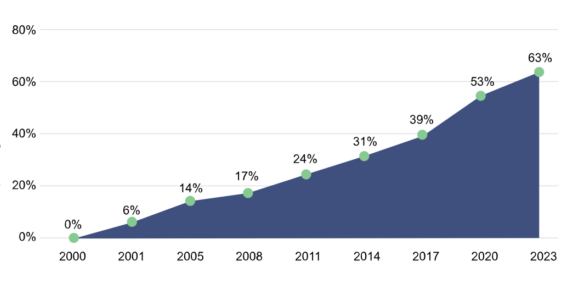
Source: Guttmacher Institute
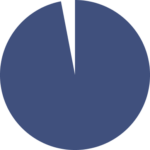
97%
patient satisfaction with medication abortion care
To help you deliver medication abortion care, we have created and curated the following regulatory guidance, training materials, and clinical resources. Learn more about the Pharmacists CARE Initiative here.
REMS INFORMATION AND RESOURCES
Danco - Mifeprex (mifepristone)
List of pharmacy locations that dispense Mifeprex (mifepristone) - Danco
Questions for Danco? Email Danco
Genbiopro - Mifepristone
Genbiopro Pharmacy Agreement Form
List of pharmacy locations that dispense mifepristone - Genbiopro
Questions for Genbiopro? Email Genbiopro
Pharmacy Specific Resources
Safe Online Pharmacies
Additional Resources
In the Media
What the Trade Associations are Saying
RELATED BLOG POSTS

Announcing the Pharmacists CARE Initiative to Increase Access to Reproductive Health Services in California
$2 Million Contract Awarded to the Pharmacists CARE Initiative to Expand Medication Abortion Services in CaliforniaBirth Control Pharmacist, in partnership with the California Pharmacists Association (CPhA) and the CPhA Foundation, is proud to announce the receipt of a transformative $2 million grant from the California Department of Health Care Access and Information. Over the next…

Misoprostol-Only Medication Abortion Regimen
Political Climate After the U.S. Supreme Court’s decision to eliminate the constitutional protections for abortion in Dobbs v. Jackson Women’s Health Organization in June 2022, access to mifepristone and abortion services in general are being threatened across the country. An ongoing anti-abortion lawsuit in Texas seeks to reverse mifepristone’s FDA approval and remove it from…

Pharmacists Can Now Dispense Mifepristone Under Updated REMS Program
What Does the January 2023 Update Mean for Mifepristone Dispensing? Mifepristone is a medication that is used to end an early pregnancy. It has been available in the United States since 2000 and is widely used as a safe and effective option for ending a pregnancy during the first 10 weeks. As of January 2023,…

Upcoming Changes to the Mifepristone REMS Program: Implications for Pharmacy Practice
Pharmacists in the community setting may soon have the opportunity to ease access to medication abortion in the United States. In the coming months, mifepristone (Mifeprex) is anticipated to have an updated Risk Evaluation and Mitigation Strategy (REMS) Program that allows dispensing through local brick-and-mortar and mail-order pharmacies. This change will integrate pharmacists into abortion…

Present and Future Pharmacist Roles in Medication Abortion Care
Although there are currently restrictions on the ways that patients can obtain a medication abortion, this many soon change and pharmacists will be an important part of access.
Medication Abortion Curriculum: A Pharmacy Student Perspective
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.

Reproductive Health During COVID: Eliminating FDA’s Burdensome Barriers to Mifepristone
What is Mifepristone? Mifepristone is the primary component in the FDA-approved regimen taken to terminate pregnancies through 10 weeks gestation and is seen as an alternative to a surgical procedure.1 Many patients view this as less invasive, allowing for more privacy and control over a personal situation. The standard oral regimen includes mifepristone 200mg followed…
This content was originally developed with the assistance and guidance of:
- American Pharmacists Association (APhA)
- National Association of Boards of Pharmacy (NABP)
- The National Alliance of State Pharmacy Associations (NASPA)
- National Community Pharmacists Association (NCPA)
- Butler University College of Pharmacy and Health Science
- Ohio State University College of Pharmacy