Blog
- All
- California
- Commentary
- Guidelines
- Indications
- Methods
- News
- Pharmacy
- Policy
- Reference
- Research
- Training
- Uncategorized
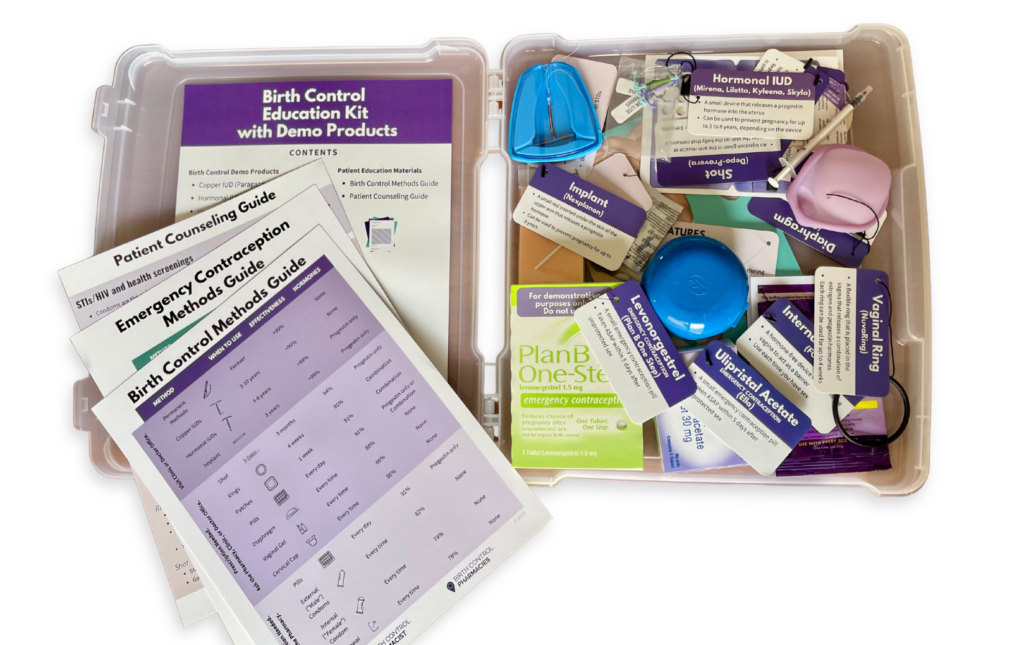
Enhancing Patient Understanding With Contraceptive Demonstration Kits
Dr. Ashley Meredith discusses how she uses the Birth Control Education Kit as a useful resource for patient counseling and engaging classroom instruction.
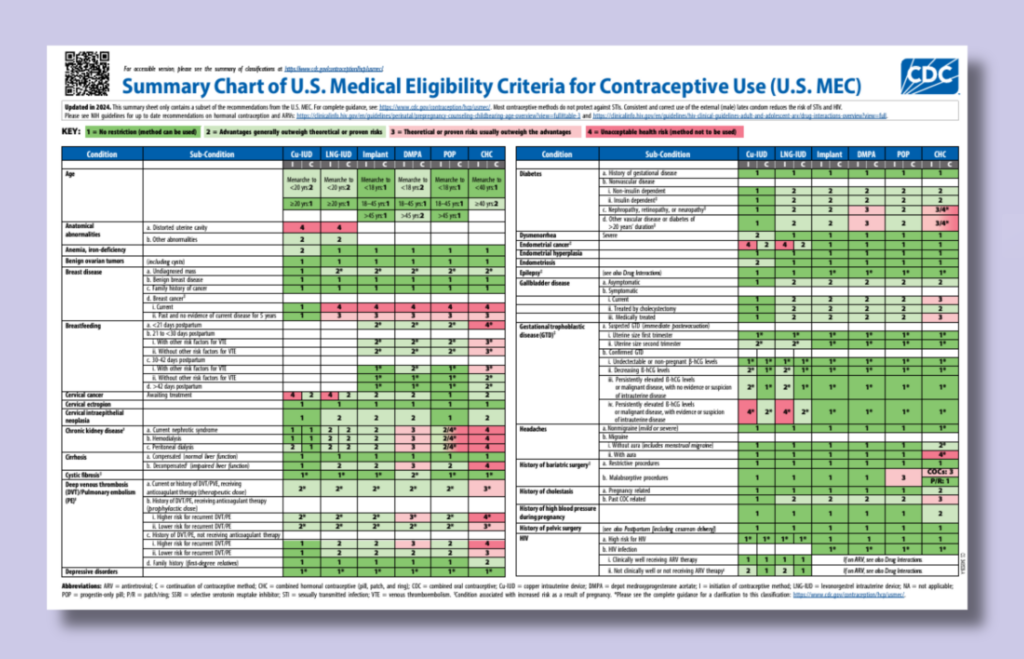
Updated CDC Contraception Guidelines
On August 6, 2024, the CDC released the 2024 U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) and the 2024 U.S. Selected Practice Recommendations for Contraceptive Use (U.S. SPR).…
Founder Reflections on 2023
As I reflect on this past year, 2023 finally brought us some wins in reproductive health. And I personally had the opportunity to contribute to landmark steps forward. 
Over-the-Counter Birth Control Pills: FDA Considering HRA Pharma’s Opill
Why Over-the-Counter Birth Control? Oral contraceptives have been around since 1960, and their risks and safety profiles have been well-studied throughout the years. According to the Guttmacher Institute, unplanned pregnancies…
How Pharmacy Students Can Advocate for Pharmacist Prescribing of Hormonal Contraception
Can pharmacy students advocate for pharmacists prescribing hormonal contraceptives? YES, that is exactly what Wilson Pace, a graduate of the University of Utah College of Pharmacy did. When Wilson heard about…
Society of Family Planning Annual Meeting Highlights
The Society of Family Planning (SFP) annual meeting was held virtually on October 1st and 2nd this year with well over one thousand attendees. For those who are not familiar…
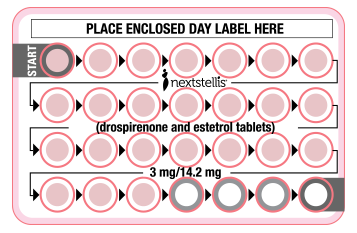
Nextstellis®: A new drug update
A new combined oral contraceptive was approved by the FDA (Nextstellis®) in April 2021.1 Nextstellis contains estetrol, an estrogen that can be manufactured from plants and that was originally…
A Primer on Reproductive Justice for Pharmacy Professionals
A pharmacy student reflects on what she learned from “Sex & Gender 101” — a gender-inclusive series designed to show learners how to take the first steps to providing care that addresses the specific needs of LGBTQI+ patients. This series includes a glossary of terms, resources for further study, and a “cheat sheet” for providers interacting with patients across the gender spectrum.
Updated Report on State Policy Efforts to Expand Access to Contraception in Pharmacies
Download free 22-page report describing the current landscape of direct access to contraception in pharmacies, state policy approaches and experiences, as well as implementation.
Sex & Gender 101
A pharmacy student reflects on what she learned from “Sex & Gender 101” — a gender-inclusive series designed to show learners how to take the first steps to providing care that addresses the specific needs of LGBTQI+ patients. This series includes a glossary of terms, resources for further study, and a “cheat sheet” for providers interacting with patients across the gender spectrum.
Levonorgestrel Intrauterine Device for Emergency Contraception
A new research study evaluates levonorgestrel IUD for emergency contraception
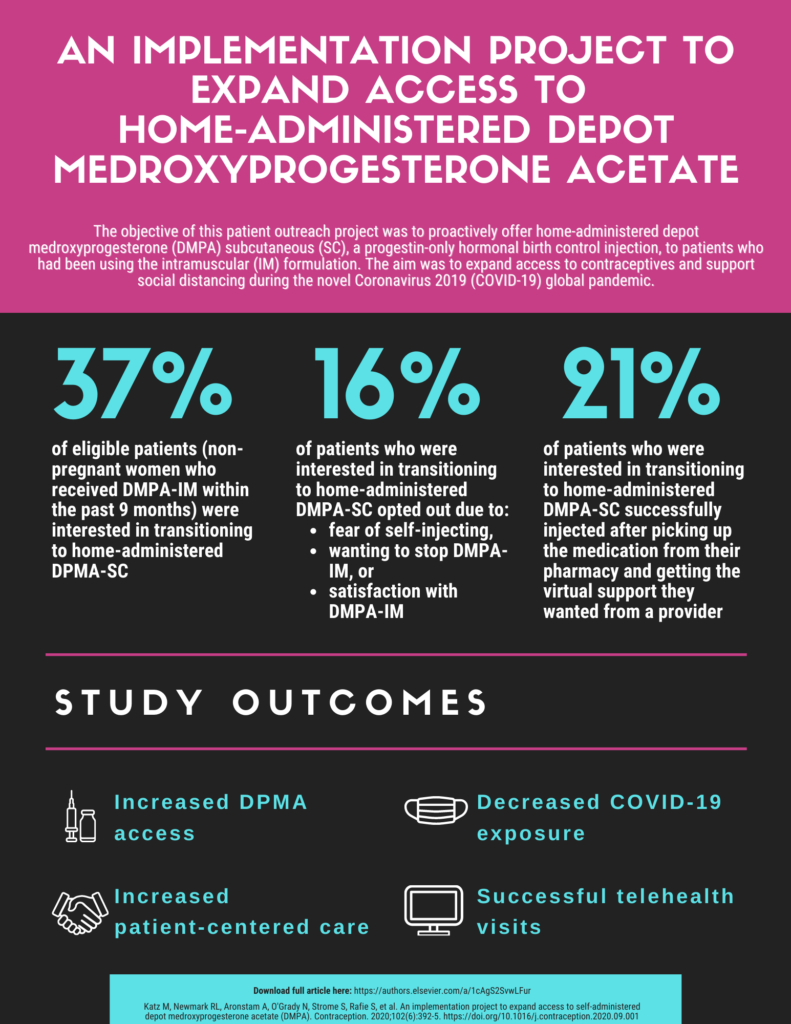
Outreach Initiative to Expand Access to Depot Medroxyprogesterone Acetate (DMPA)
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Webinar Introduces Pharmacists to New Hormonal Contraceptives
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Webinar Equips Pharmacists to Provide Contraception Care During COVID-19
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Putting Policy into Practice: Contraception Care in San Francisco Pharmacies
What makes some pharmacies more successful than others at implementing pharmacist-prescribed contraception care? To answer this question, we conducted a study to determine the extent of hormonal contraceptive prescribing, also referred to as furnishing in California, among San Francisco community pharmacies, and identify the factors that led to successful implementation.
Reducing Maternal Mortality in the United States through Collaboration
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
New Webinar Prepares Pharmacists to Provide Reproductive Health Services and Referrals
New training program by Birth Control Pharmacist and Provide includes pharmacy best practices to combat stigma and communication guides and online resources to help connect patients with local resources.
Meet Phexxi – A New Non-Hormonal Contraceptive Gel
About the Product Lactic acid, citric acid, and potassium bitartrate (Phexxi, Evofem Biosciences) is a prescription combination, non-hormonal contraceptive gel approved by the FDA in May 2020. The vaginal gel…
Can Contraceptives be Vegan? Important Considerations for Vegan Patients
The Vegan Society defines veganism as “a way of living which seeks to exclude, as far as is possible and practicable, all forms of exploitation of, and cruelty to, animals…
Measuring Blood Pressure: An Important Prerequisite to Prescribing Hormonal Contraception
Why is it important to measure blood pressure before prescribing hormonal contraception? Combined hormonal contraceptives (CHCs) are a relatively safe and effective method for your patients in preventing pregnancy and…















Continuing Contraception Care After Trump’s Orders
In light of recent actions over the last two weeks with our new administration, I wanted to provide updates and guidance to my pharmacist peers regarding reproductive health care.On Friday…